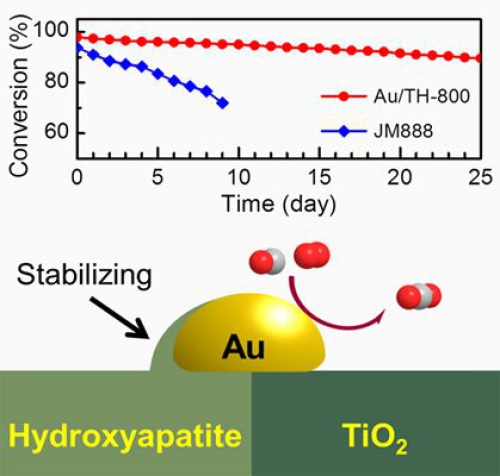
We have successfully synthesized an ultrastable and highly active Au nanocatalyst (Au/TiO2-HAP) by tuning the Strong Metal-Support Interaction (SMSI) between Au and hydroxyapatite (HAP). This catalyst demonstrated excellent durability that outperforms a commercial Three Way Catalyst (TWC) JM888 for CO oxidation under simulated practical conditions.
During the past decades, supported Au nanocatalysts have attracted intensive and increasing interest because of their unique catalytic properties for a variety of reactions. Their poor thermal stability, however, presents a major barrier to the practical applications. Therefore, it still remains a formidable challenge to fabricate ultrastable Au nanocatalysts with high activity for practical applications.
The Au NPs located at the TiO2/HAP interfaces (Image by TANG Hailian and QIAO Botao)
We discovered that Au nanoparticles (NPs) could form SMSI with HAP upon high-temperature calcination and result in the encapsulation of Au NPs which improves their sintering resistance significantly (J. Am. Chem. Soc. 2016, 138, 56-59). However, the full encapsulation reduces their activity due to the coverage of the active sites. To resolve this problem, the researchers manipulated the SMSI between Au and HAP (i.e., the degree of encapsulation) by incorporating TiO2 into the HAP support. It was found that this catalyst possessed high activity and goods intering resistance after calcination at 800oC for a variety of reactions.
It also demonstrated excellent durability that outperforms a commercial TWC for CO oxidation under simulated practical conditions. Systematic studies show that the Au NPs primarily located at the interfaces between TiO2 and HAP makes the Au NPs partially covered by a thin layer of the HAP. The SMSI contributed to the excellent stability and anti-sintering property of the catalyst, and the partially exposed to TiO2 contributed to the high activity of the catalyst. This work suggests the great potential of supported Au catalysts for practical applications. Moreover, this work may provide a new strategy to develop ultrastable Au nanocatalysts with high activity by designing and tuning the SMSI.
This work was published on Angew. Chem. Int. Ed. (http://dx.doi.org/10.1002/anie.201601823). Prof. Masatake Haruta, the pioneer in gold catalysis, highlighted this work with a short comment entitled as “Ultrastable nanogold catalyst—on the way going to practical application” (http://www.cjcatal.org/CN/10.1016/S1872-2067(16)62526-3). This work was financially supported by the National Natural Science Foundation of China. (Text and Image by TANG Hailian and QIAO Botao)