Nowadays, the scarcity of fresh water resources and the ever-growing environmental pollution have been attracting increased concern. The Fenton-like process has been widely investigated due to its high efficiency in removing persistent organic contaminants by in-situ production of SO4-·or HO· radicals. However, limited mechanism understanding hinders any significant advances in Fenton chemistry. In the past two years, we have made a series of progress in investigating the mechanism of Fenton-like reaction, by using Mössbauer technique determine the coordination environment, spin state and oxidation state of iron ions.
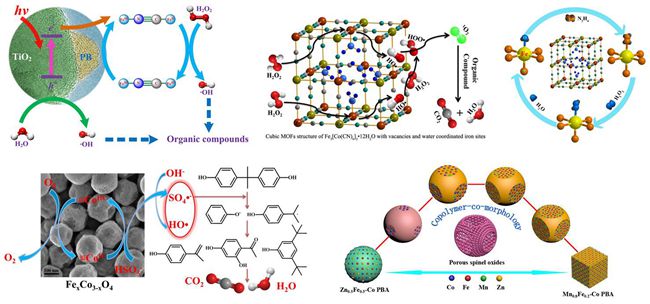
In 2014, we developed a series of Prussian blue/TiO2 (PB/TiO2) nanocomposites as heterogeneous photo-Fenton catalyst to increase the FeII recovery in degrading organic contaminants in water. The Mössbauer spectra provided evidence that the excited electrons of TiO2 by UV irradiation could accelerate the cycle of low spin FeIII to FeII in PB and then led to the higher activity (Catal. Sci. Technol., 2015, 5, 504-514. doi: 10.1039/C4CY00947A).
Then, two kinds of Fe-Co Prussian blue analogues (Fe-Co PBAs) with different iron valence state, Fe3[Co(CN)6]2·12H2O and Fe[Co(CN)6]·2H2O, were developed as novel photo-Fenton catalysts for in-depth investigation of the heterogeneous Fenton reaction mechanism. The efficient redox cycling of iron species in the Fe-Co PBAs photo-Fenton process was deeply explored by Mössbauer spectroscopy. The excellent photo-Fenton activities of these Fe-Co PBAs were ascribed to the existence of highly dispersed water coordinated iron sites and abundant vacancies in the metal-organic-frameworks. In collaboration with Prof. Hongxian Han from the State Key Laboratory of Catalysis, singlet oxygen (1O2) produced indirectly from HOO· and HO· was found to participate directly in the degradation of RhB (Appl. Catal. B 2016, 181, 788-799. doi:10.1016/j.apcatb.2015.05.033 2015/07-09 Top 25 Hottest Articles: 25/25).
To further study the reaction intermediates and pathway of active iron species during the Fe-Co PBA catalyzed Fenton-like process, hydrazine (Hz) was newly introduced to enhance the oxidation performance of the Fenton system. Combining the XPS and Mössbauer spectroscopy results, the Hz coordinated iron site (H2NH2N-Fe), which is evolved from the original water coordinated iron site (H2O-Fe), was identified as a more active site which largely increased the reaction rate (Catal. Commun., 2016, doi: 10.1016/j.catcom.2016.01.004).
Mechanism of Fenton-like Reaction by Using Mössbauer Technique (Image by Xuning LI)
Based on the deeply understanding the Fenton-like reaction mechanism as well as the structural features of the Fe-Co PBAs, we successfully prepared a series of porous FexCo3-xO4 nanocages via a facile strategy by heating FeyCo1-y-Co PBAs nanospheres with tunable size and morphology. The prepared FexCo3-xO4 nanocages were found to be efficient to activate PMS into radicals, which could further degraded BPA to small organic compounds or even inorganic carbon forms. Mössbauer and XPS techniques were applied to illustrate the catalytic mechanism and B-site CoII on the surface of FexCo3-xO4 nanocages was determined as the main factor for PMS activation (Appl. Catal. B 2016, 181, 788-799. doi:10.1016/j.apcatb.2015.08.050 2015/07-09 Top 25 Hottest Articles: 15/25).
Meanwhile, we successfully synthesized series of MnyFe1-y-Co PBAs nano-dices with well-controlled morphology and composition through simply modulating the relative content of Mn. Based on the fact of that three series of MyFe1-y-Co PBAs (M = Co, Mn and Zn) with well-controlled morphology have been successfully prepared through this novel and scalable strategy, a “copolymer-co-morphology” conception was unprecedentedly proposed. In collaboration with Prof. Luhua Jiang from Dalian National Laboratory for Clean Energy, the octahedral-site MnIII/MnIV content in MnxFe1.8-xCo1.2O4, mainly determined by sensitive 57Fe Mössbauer and XPS techniques, was discovered to be directly correlated with the oxygen reduction/evolution reaction (ORR/OER) activity (Nanoscale, 2016, doi: 10.1039/C5NR07193C).
The series of research not only opens a new way for the general use of PBAs in photo-Fenton process, but also gives a deeper insight into the heterogeneous Fenton reaction mechanism which is nowadays very limited. In addition, the “copolymer-co-morphology” conception will be beneficial for morphologically controlled syntheses of other types of CPs and spinel oxides.
The above-mentioned research work has been financially supported by the National Natural Science Foundation of China and the Chinese Academy of Sciences Visiting Professorships for Senior International Scientists. (Text and Image by Xuning LI)