The development of efficient non-precious metal catalysts (NPMCs) can help reduce the cost of fuel cells and accelerate their commercialization. At present, non-precious metal catalysts generally suffer from poor stability in proton exchange membrane fuel cells, but there is still a lack of understanding of the degradation mechanism at molecular scale.
Recently, a research group led by Prof. SUN Gongquan and Prof. WANG Suli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) collaborated with our Center revealed the degradation mechanism of Fe-N-C-type NPMCs by Mössbauer spectroscopy.
This work was published in Applied Catalysis B: Environmental on March 4.
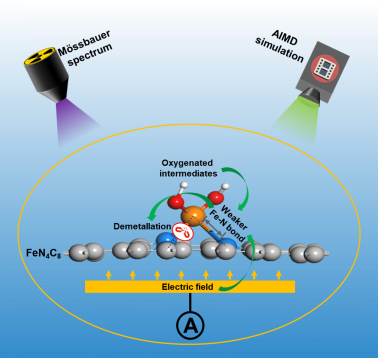
The demetallation varied by the structure of active site was investigated using end-of-test (EoT) and in-situ Mössbauer spectroscopy. It’s revealed that D1 is the main active site for Oxygen Reduction Reaction(ORR) and its demetallation is responsible for the degradation of Fe-N-C. The rapid initially but slow afterward degradation can be explained by the different activity and stability of various FeN4 sites.
The demetallation due to structural evolution during ORR was investigated using ab initio molecular dynamics (AIMD) simulations. It’s revealed that the adsorption of oxygenated intermediate and the motivation of electric field can further weaken the Fe-N bond in FeN4, leading to more severe demetallation. The faster degradation of Fe-N-C under higher potential can be explained by the stronger adsorption and field intensity.
This work provides the insight into the degradation mechanism of Fe-N-C at the molecular level and the directional guidance for the future development of stable NPMCs in fuel cell devices.
This work was supported by National Natural Science Foundation of China, and Postdoctoral Science Foundation of China. (Text by Xu Xinlong)