NiFe (hydr)oxides have attracted significant attention as metal oxide-based electrocatalysts for the oxygen evolution reaction (OER) due to their remarkable efficiency and stability. Understanding their high catalytic performance, along with clarifying the specific roles of Fe and Ni ions in the OER process, is of great importance. Iron and nickel may make unique contributions to catalytic processes by altering valence states, forming oxygen intermediates, or promoting charge transfer during the reaction process. Clarifying these fundamental issues can further improve the potential to optimize these catalysts for better performance, opening the new avenue to develop more cost-effective OER catalysts for clean energy technologies.
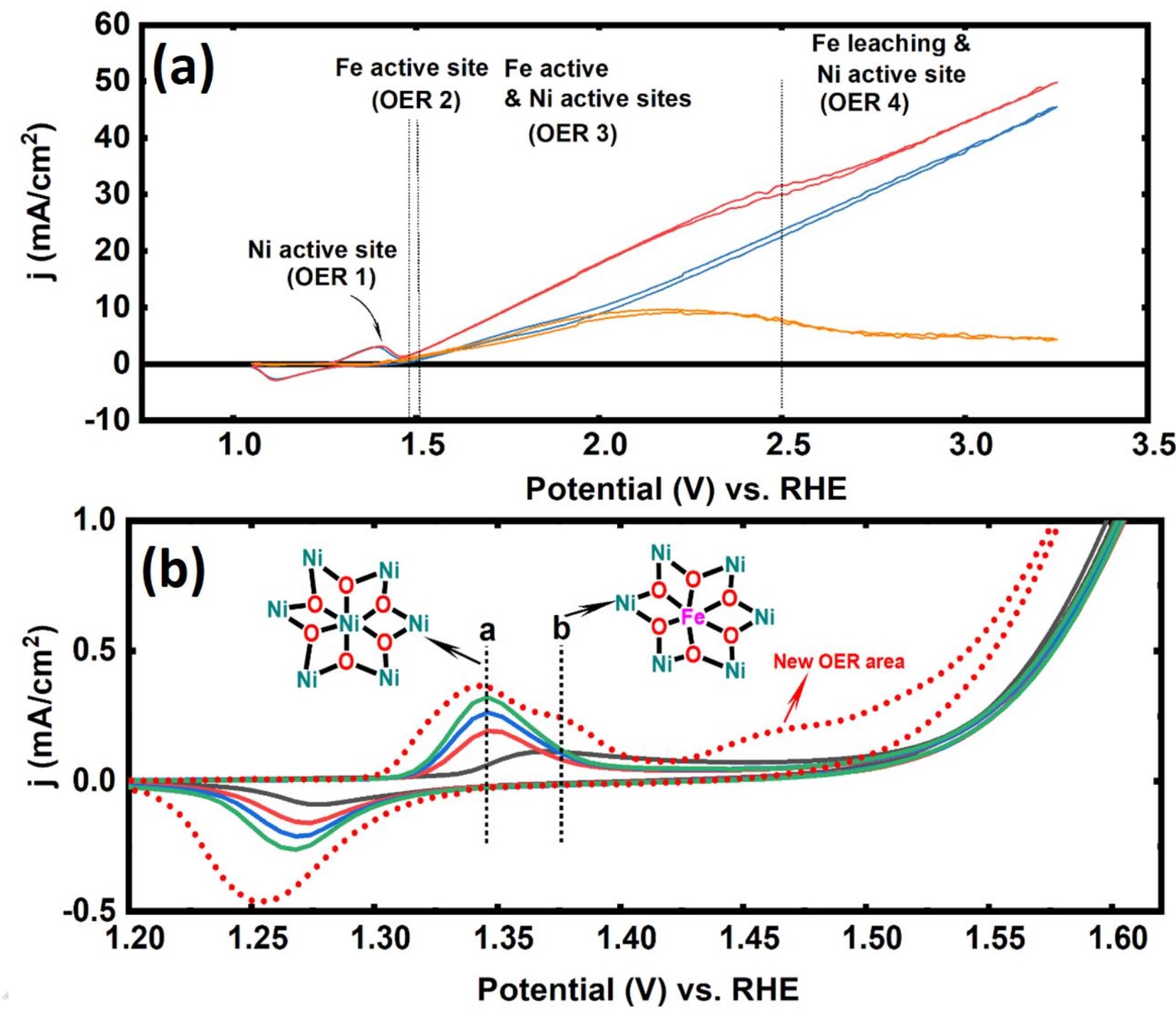
Fig. 1. (a) CVs for a pure Ni wire before and after adding Fe salt to a Ni/Fe-free electrolyte. The subtraction (orange) of the CV curve in the absence of the Fe salt from the CV curve in the presence of the Fe salt. (b) CVs of a large Ni foam before the first, second, third, and fourth CV and after adding trace Fe.
In this study, we conducted a comprehensive investigation of the roles of high-valent iron in NiFe (hydr)oxide OER catalysts in alkaline media. Using a range of in situ/operando techniques, including visible spectroscopy, surface-enhanced Raman spectroscopy (SERS), X-ray absorption spectroscopy and Mössbauer spectroscopy, we tried to elucidate the dynamic changes and mechanisms occurring during the OER. It is surprised that high-valent iron ions exhibit remarkable stability within the NiFe oxide structure, even after the OER process over many days. When performing the OER on nickel hydroxides treated with iron salts, no high-valent iron species were detected. Experimental results indicate that the oxidation of bulk iron to high-valent states (similar to nickel ions) is related to charge accumulation rather than direct participation in OER catalysis. These findings support our hypothesis that a small amount of surface high-valent iron ions actively participates in the OER process, while bulk high-valent iron primarily serves for charge accumulation. Our study also provides new insights into the oxygen evolution regions and associated active sites at different overpotentials. The strategic synthesis and detailed analysis of NiFe (hydr)oxides allowed us to propose two distinct iron species, surface iron and bulk iron, further highlighting the importance of interactions between iron and nickel, and demonstrating that the controlled incorporation of iron can optimize catalytic performance.
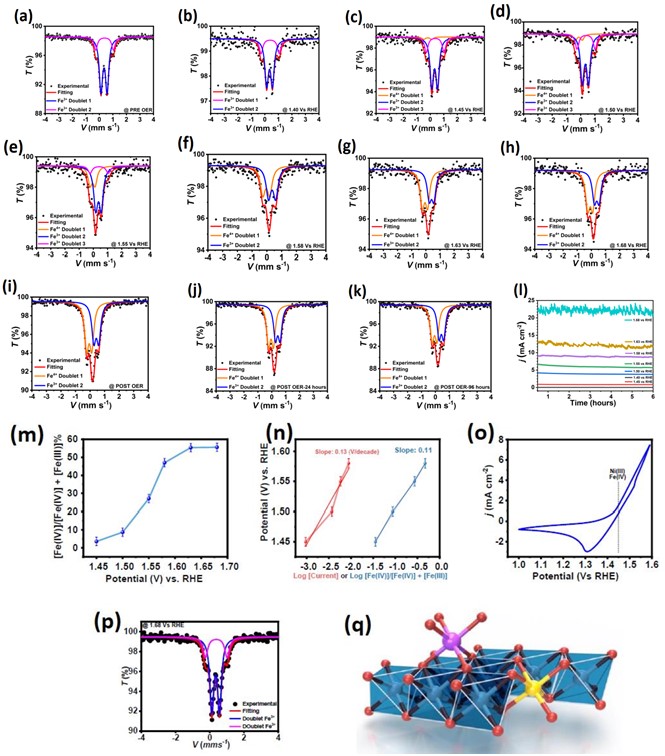
Fig. 2. In-situ/operando Mössbauer spectroscopic analysis of NiFe (hydr)oxide at different potentials or after OER and relatedchronoamperometry.
The related findings were published under the title "A Hypothesis on the Function of High-Valent Fe in NiFe (Hydr)oxide in the Oxygen-Evolution Reaction" in Angew. Chem. Int. Ed. This work was supported by the Iran National Science Foundation, Sharif University of Technology, Institute for Advanced Studies in Basic Sciences, the National Natural Science Foundation of China (22350410386, 22375200, U22A20394, 21961142006), the Chinese Academy of Sciences International Partnership Program (028GJHZ2023097GC), and the China Postdoctoral Science Foundation Fellowship Program (GZC20232590).
Relevant link: https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202418798