Mössbauer spectroscopy is the science of studying the behavior of electrons outside the nucleus. It has high energy resolution and can observe the hyperfine interactions of Mössbauer nuclides, and characterize the phase, valence state, coordination structure and magnetism of the Mössbauer elements in samples, such as iron and tin.
The in situ/operando electrochemical Mössbauer spectroscopy can be used to explore the changes of electronic, structural and magnetic properties of the electrocatalytic materials under real working conditions. It is simple to operate and sensitive to detect. It is a powerful tool to study the structure-activity relationship and mechanism of electrocatalyst in electrochemical reaction.
Recently, our Center developed a set of in-situ/operando electrochemical 57Fe and 119Sn Mössbauer instrument. It can be used to dynamically observe the evolution of active phases of iron-based and tin-based catalysts in electrochemical reactions, and reveal the intrinsic nature of the electrocatalysts.
In this instrument, single line Mössbauer radiation source used keV level gamma ray, which could reduce attenuation in electrolyte and obtain satisfied signal-to-noise ratio. Electrocatalyst sample only needs to be evenly coated on carbon paper or carbon cloth before the test, and then the electrochemistry and Mössbauer spectroscopy could be simultaneously conducted.
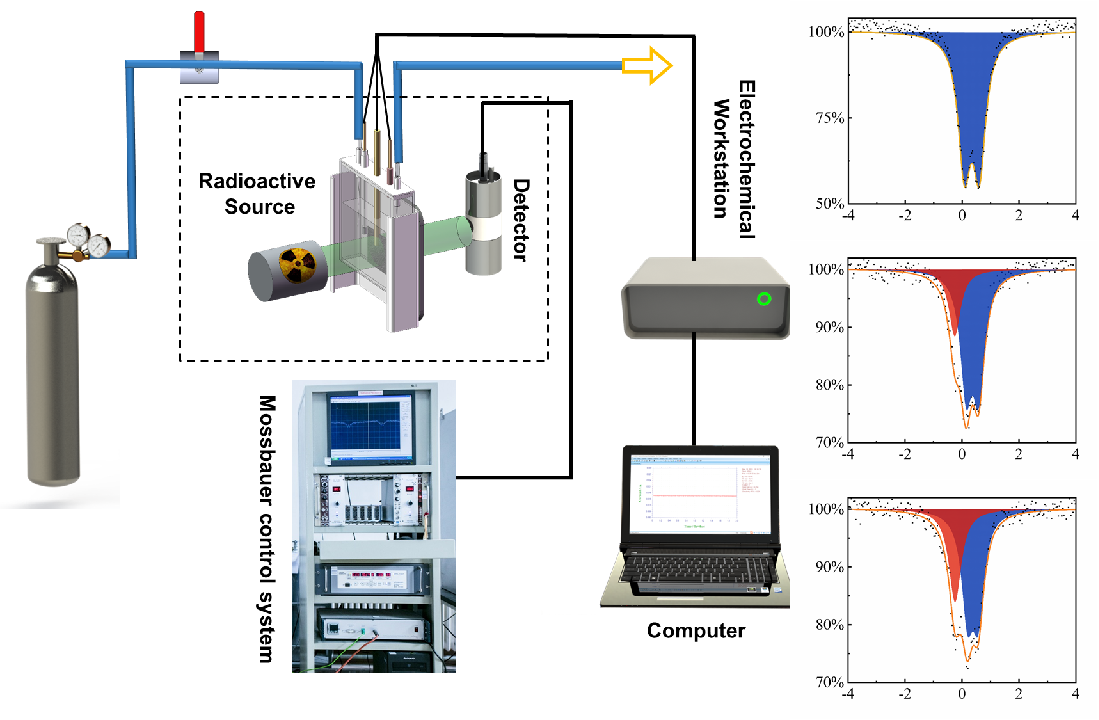
Schematicallys how the self-developed in-situ/operando electrochemical 57Fe and 119Sn Mössbauer instrument and practical example of three spectra of the topotactically constructed nickel-iron (oxy)hydroxide electrocatalyst with abundant Fe4+ species generated during water oxidation reaction (Image by KUANG Zhichong)
This instrument had been applied in the oxygen evolution reaction (OOR), oxygen reduction reaction (ORR) and carbon dioxide reduction reaction (CO2RR). The test results showed that the obtained spectra had satisfied signal-to-noise ratio.
For the in-situ/operando testing, samples may be prepared using enriched isotopes 57Fe or 119Sn if the iron or tin content of the sample is too small.
This instrument provides a high level of research tool for the preparation of highly efficient catalysts of electrolytic water and carbon dioxide reduction. (Text by KUANG Zhichong)